Diagnosing Sleep Apnea: Key Factors to Consider in Home Sleep Apnea Testing Solutions
Diagnosing Sleep Apnea: Key Factors to Consider in Home Sleep Apnea Testing Solutions
Author: Antti Näykki, Director of Sleep Apnea Product Line, Medical Technologies, Bittium
26 percent of adults between the ages of 30 and 69 worldwide suffer from obstructive sleep apnea (OSA), the most common type of sleep-related breathing disorders. The risks and consequences should not be underestimated. Intelligent medical technology enables outpatient examinations through Home Sleep Apnea Testing (HSAT) solutions. However, when choosing an appropriate solution it is important to consider various key parameters.
Diagnostic Challenges
For a long time, monitoring patients in a sleep laboratory was the only reliable way to diagnose sleep disorders and it is still a very reliable method. Disadvantages of this examination method are the discomfort and unfamiliar environment for the patient as well as the high costs. For this reason, some patients might even be reluctant to undergo such an examination.
So far, solutions for outpatient examinations have been largely too imprecise because they could not record all the necessary biosignals. A new generation of intelligent solutions for Home Sleep Apnea Testing can make life significantly easier for medical staff and patients. It is also estimated to be approximately seven times more cost-effective than an examination in the sleep laboratory. The prerequisite is in any case that the solutions capture the necessary biosignals for an accurate diagnosis.
AASM Classification
The American Academy of Sleep Medicine (AASM) has taken this development into account and created a classification in which supervised and unsupervised examinations in sleep laboratory are listed as types 1 and 2. The new HSAT (Home Sleep Apnea Testing) solutions that cover the required parameters are classified as type 3. Diagnostic solutions or approaches in which fewer parameters are recorded are listed as type 4 or classified as “other´.
The most important factors for the suitability of an HSAT solution
To ensure an accurate diagnosis (type 3 of the AASM classification), the solution should measure at least the following parameters:
- ECG or heart rate
- Oxygen saturation
- At least two respiratory parameters (such as respiratory airflow and respiratory movement)
- The solution needs to have medical certification.
When it comes to patient convenience, criteria to consider include:
- Easy operation of the device to avoid complicated instructions and possible operating errors.
- Comfortable use, for which small and lightweight devices without numerous cables are a great advantage.
For the medical staff who make the diagnoses, there are additional factors that affect the effective use of the solution:
Data management: Safe and simple data management also plays an important role for clinics and medical service providers. End-to-end solutions enable integration into online platforms for evaluation, analysis, reporting and management of the corresponding medical and patient data such as the Bittium MedicalSuite™.
Offline and online functions: There are HSAT solutions that work offline and collect data for evaluation in the device, as well as solutions that, with the patient’s consent, transfer the data directly to the supervising medical personnel – or support both usage options. Remote support with direct data transmission offers advantages for prompt diagnosis. However, when it comes to data transmission it is particularly important that the device meets the highest standards for information security.
Data analysis: To make the work of medical staff easier, the latest generation of solutions offers an option for pre-analyzing the measurement data with the help of artificial intelligence. In this way, important patterns for making a diagnosis can be identified more quickly.
Bittium Respiro™
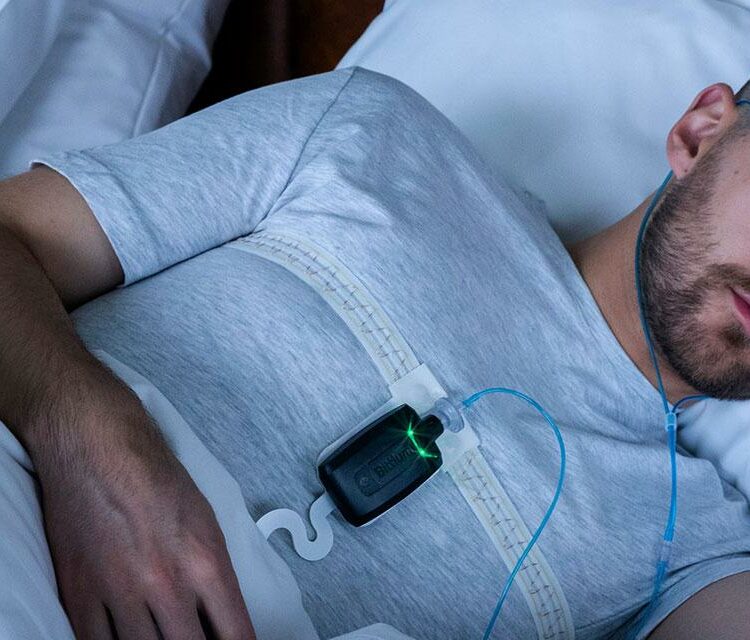
Take a look at our new solution Bittium Respiro™, a medical device intended to record standard PG level data according to AASM recommendations and analyze typical sleep-related breathing disorders, such as obstructive sleep apnea, central sleep apnea, mixed sleep apnea, hypopnea and Cheyne-Stokes breathing. Bittium Respiro Analyst™ software provides a web-based intuitive user interface which utilizes artificial intelligence to accelerate analysis work. The software pre-analyses the massive amount of measurement data and converts it to a more visual and informative format. This makes it easier and quicker for healthcare personnel to perform further analysis and diagnosis. Bittium Respiro™ is designed to be compliant with Medical Devices Regulation (MDR) and will be available to the market during Q3/2021.
Antti Näykki, Director of Sleep Apnea Product Line, Medical Technologies, Bittium
Since joining Bittium in April 2019, Antti has put his mind and effort in business development and executing strategic projects in R&D and medical technologies. Antti has long experience in sustainable business development and management. In his current role as a leader of Bittium’s sleep apnea product line, he has held a key position in combining his experience in business management with innovative concepts, while working close to customers and partners.
